A QM/MM MD study of the pH-dependent ring-opening catalysis and lid motif flexibility in glucosamine 6-phosphate deaminase†
Abstract
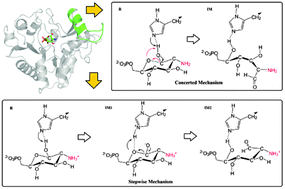
The glucosamine 6-phosphate deaminase (NagB), which catalyzes the conversion of D-glucosamine 6-phosphate (GlcN6P) into D-fructose 6-phosphate (F6P) and ammonia, determines the final metabolic fate of N-acetylglucosamine (GlcNAc). Here using state-of-the-art ab initio QM/MM MD simulations, we have explored the plausible mechanisms for the enzymatic ring-opening of GlcN6P in the basic environment. Two different proton-shuttle mechanisms have been proposed. Calculations show that the protonated state of the amino group in the substrate dominates the concerted and stepwise catalytic pathways and a catalytic triad plays an important role in mediating the proton transfer and the resulting ring-opening process. The free energy barrier for the rate-determining step in the low-energy stepwise reaction is 17.9 kcal mol−1. In acidic solution, the lid motif prefers a closed state while it always stays in the open state in basic solution upon substrate binding, which is basically dominated by the protonated state of the residue His145.

 Please wait while we load your content...
Please wait while we load your content...