CO dissociation on magnetic Fen clusters
Abstract
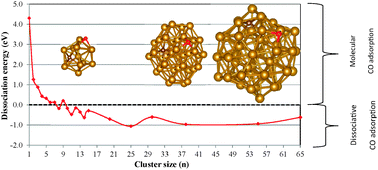
This work theoretically investigates the CO dissociation on Fen nanoparticles, for n in the range of 1–65, focusing on size dependence in the context of the initial step of the Fischer–Tropsch reaction. CO adsorbs molecularly through its C-end on a triangular facet of the nanoparticle. Dissociation becomes easier when the cluster size increases. Then, the C atom is bonded to a square facet that is generated as a result of the adsorption if it does not yet exist in the bare cluster, while the O atom is adsorbed on a triangular facet. In the most stable situation, the two adsorbed atoms remain close together, both having in common one shared first-neighbor iron atom. There is a partial spin quenching of the neighboring Fe atoms, which become more positively charged than the other Fe atoms. The shared surface iron atom resembles a metal-cation from a complex. Despite the small size of the iron cluster considered, fluctuations due to specific configurations do not influence properties for n > 25 and global trends seem significant.

 Please wait while we load your content...
Please wait while we load your content...