A σ-hole interaction with radical species as electron donors: does single-electron tetrel bonding exist?
Abstract
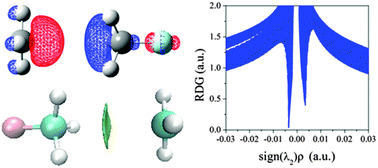
A single-electron tetrel bond was predicted and characterized in FXH3⋯CH3 (X = C, Si, Ge, and Sn) complexes by performing quantum chemical calculations, where the methyl radical acts as the Lewis base and the σ-hole on the X atom in FXH3 as the Lewis acid. The interaction between the methyl radical and FXH3 is characterized by a red shift of F–X stretching frequency. The strength of the tetrel bond becomes stronger by not only increasing the atomic number of the central atom X (X = C, Si, Ge, and Sn) but also by enhancing the electron-withdrawing ability of substituents in the Lewis acid. The energy decomposition analysis highlights the importance of the electrostatic interaction in the formation of the tetrel bond, although the dispersion part is also non-negligible for the weak tetrel bond. There is a competition between the formation of single-electron tetrel bonds and hydrogen bonds for the complexes composed of the methyl radical and CNCH3 or NCCH3. Furthermore, the single-electron tetrel bond exhibits the cooperative effect not only with the hydrogen bond in the complex of NCH⋯NCCH3⋯CH3, but also with the conventional tetrel bond in NCCH3⋯NCCH3⋯CH3.

 Please wait while we load your content...
Please wait while we load your content...