The effect of atomic ions on model σ-hole bonded complexes of AH3Y (A = C, Si, Ge; Y = F, Cl, Br)†
Abstract
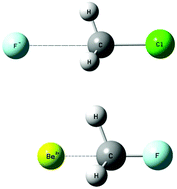
A computational study of ionic X⋯AH3–Y complexes (X = F−, Cl−, Br−, Li+, Be2+; A = C, Si, Ge; Y = F, Cl, Br) predicted optimized structures which are held together by a combination of attractive forces, including ion–dipole and ion–σ-hole electrostatic interactions, and polarization forces. The trends (with variation in the halogen Y) for selected properties were rationalized by considering the electron density shifts due to the ion's electric field. Although it has been found previously that the trends for binding energies in neutral complexes follow the sigma-hole strength, the present study found that the dependence on the dipole polarizability of the A–Y bond can explain the trends for binding energies in these more strongly bound ionic complexes.

 Please wait while we load your content...
Please wait while we load your content...