The local structure of PdxCe1−xO2−x−δ solid solutions†
Abstract
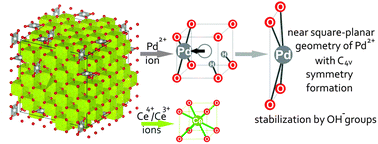
PdxCe1−xO2−x−δ solid solutions, which are highly efficient catalysts for the low-temperature oxidation of carbon monoxide, were examined using a set of structural (XRD-PDF, HRTEM, XRD) and spectral (XPS, Raman spectroscopy) methods in combination with quantum-chemical calculations. A comparison of the experimental results and pair distribution function (PDF) modeling data enabled reliable verification of the model of non-isomorphic substitution of Ce4+ ions by Pd2+ ions in PdxCe1−xO2−x−δ solid solutions. Palladium ions were shown to be in a near square planar environment with C4v symmetry, which is typical for Pd2+ ions. Such a near square planar environment was revealed by Raman spectroscopy due to the appearance of the band at ω = 187 cm−1, which corresponds to the A1 vibrational mode of Pd2+ ions in [PdO4] subunits. The binding energy of Pd3d5/2 (Eb(Pd3d5/2)) for the Pd2+ ion in the CeO2 lattice is 1 eV higher than that of Eb(Pd3d5/2) for PdO oxide due to a decrease in the Pd–O distances and the formation of more ionic bonds because of the displacement of Pd2+ ions with respect to the position of Ce4+ ions in the fluorite structure. Five structural models of solid solutions are considered in this work. As demonstrated by the DFT calculations, the most realistic model is based on the displacement of palladium ions leading to a near square planar PdO4 environment, which includes water molecules stabilizing the region of anion vacancies in their dissociated state as two hydroxyl groups. The introduction of water molecules in the composition of the PdxCe1−xO2−x−δ solution leads to a decrease in the formation energy and to additional stabilization of palladium in the CeO2 matrix. The formation of PdxCe1−xO2−x−δ solid solutions is accompanied by the dispersing effect caused by distortions of the fluorite structure induced by Pd2+ ions. The coprecipitation method, which allows Pd2+ ions to be introduced at the stage of fluorite structure formation, was demonstrated to be the optimal method for the synthesis of a homogeneous PdxCe1−xO2−x−δ solid solution.

 Please wait while we load your content...
Please wait while we load your content...