Structures of ionic liquid–water mixtures investigated by IR and NMR spectroscopy
Abstract
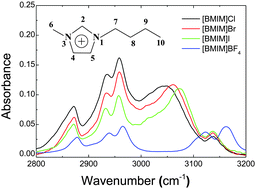
Imidazolium-based ionic liquids having different anions 1-butyl-3-methylimidazolium ([BMIM]X: X = Cl−, Br−, I−, and BF4−) and their aqueous mixtures were investigated by IR absorption and proton NMR spectroscopy. The IR spectra of these ionic liquids in the CHx stretching region differed substantially, especially for C–H bonds in the imidazolium ring, and the NMR chemical shifts of protons in the imidazolium ring also varied markedly for ILs having different anions. Upon the introduction of water to screen the electrostatic forces and separate the ions, both IR and NMR spectra of [BMIM]X (X = Cl−, Br−, I−) showed significant changes, while those of [BMIM]BF4 did not change appreciably. H–D isotopic exchange rates of C(2)–H in [BMIM]X–D2O mixtures exhibited an order: C(2)–H⋯Cl > C(2)–H⋯Br > C(2)–H⋯I, while the C(2)–H of [BMIM]BF4 was not deuterated at all. These experimental findings, supported by DFT calculations, lead to the microscopic bulk configurations in which the anions and the protons of the cations in the halide ionic liquids have specific, hydrogen-bond type of interaction, while the BF4− anion does not participate in the specific interaction, but interacts less specifically by positioning itself more above the ring plane of the imidazolium cation. This structural change dictated by the anion type will work as a key element to build the structure–property relationship of ionic liquids.

 Please wait while we load your content...
Please wait while we load your content...