Absorption and fluorescence signatures of 1,2,3-triazole based regioisomers: challenging compounds for TD-DFT†
Abstract
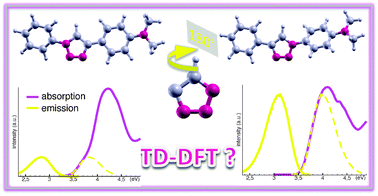
In the continuous quest for improving TD-DFT methodologies as a tool to predict the photophysical features of solvated chromophores, we investigate two model regioisomers based on the 1,2,3-triazole moiety. Starting from their experimental absorption and emission spectra, key energy differences highlighting the main trends between the two isomers are extracted and used to gauge the accuracy of several levels of theory. RI-CC2 and EOM-CCSD calculations allow us to ascertain that the low energy spectra are not linked to double excitations. In a vacuum, none of these methods nor any of the implemented TD-DFT levels of theory, ranging from global hybrids (PBE0, B3LYP) to range-separated functionals without (CAM-B3LYP, ωB97X) or with dispersion corrections (ωB97X-D), are able to capture the key features that differentiate the two chromophores. Accounting for solvent within a specific PCM model allows us to recover experimental trends, but the dramatic changes occurring when moving from toluene to THF and/or when using different PCM approaches (LR, cLR, SS) suggest that this agreement is probably fortuitous. Even if the ωB97X-D functional combined with the SS-PCM scheme leads to quantitative agreement with experiment, TD-DFT results obtained for 1,2,3-triazole based chromophores need to be treated with caution. We also show that the SS-PCM approach may be useful to test current and novel exchange–correlation functionals against the charge transfer failure.

 Please wait while we load your content...
Please wait while we load your content...