Theoretical design of molecular grippers for anion recognition based on subporphyrazines and subphthalocyanines†
Abstract
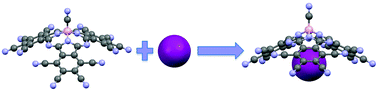
Subphthalocyanines (SubPcs) are 14 π-electron aromatic macrocycles containing three 1,3-diiminoisoindol units N-fused around a central boron atom. Their particular concave π-extended structure makes them potentially useful in fields related to optoelectronics and molecular electronics. Also, their structure makes them potential receptors of complementary convex-shaped molecules. Inspired by the properties of subphthalocyanines and by previous studies about the possibility of obtaining neutral receptors composed of aromatic rings that can bind anions, we performed a theoretical study to analyze the ability of subphthalocyanines and some SubPc analogues to capture anions by non-covalent interactions. This may be relevant, since they can produce a variety of chromogenic reagents for anion detection that are not destroyed in the detection process, since the non-covalent interactions are weak enough to reverse the procedure. We characterized complexes formed between several SubPc derivatives and different anions, and also studied the influence of this process on the optical properties of these molecules. The stabilization trend reflected by the energetic results and the changes shown in the absorption wavelengths of the host molecule under complexation confirm our hypothesis about the possibility of these kinds of molecules being used as chromogenic reagents for anion capture, even in solvents with a large dielectric constant like water.

 Please wait while we load your content...
Please wait while we load your content...