Distinguishing Förster resonance energy transfer and solvent-mediated charge-transfer relaxation dynamics in a zinc(ii) indicator: a femtosecond time-resolved transient absorption spectroscopic study†
Abstract
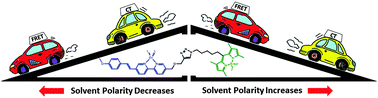
A bifluorophoric molecule (1) capable of intramolecular Förster Resonance Energy Transfer (FRET) is reported. The emission intensity of the FRET acceptor in 1 depends on the molar absorptivity of the donor, which is a function of zinc(II) complexation. The FRET dynamics of [Zn(1)](ClO4)2 is characterized by femtosecond time-resolved transient absorption spectroscopy. The solvent-mediated relaxation of the charge-transfer (CT) state of the isolated donor and the FRET process of the donor–acceptor conjugate are on similar time scales (40–50 ps in CH3CN), but distinguishable by the opposite solvent polarity dependency. As the solvent polarity increases, the efficiency of Columbic-based FRET is reduced, whereas CT relaxation is accelerated. In addition to revealing a method to distinguish CT and FRET dynamics, this work provides a photophysical foundation for developing indicators based on the FRET strategy.

 Please wait while we load your content...
Please wait while we load your content...