The interaction of gold and silver nanoparticles with a range of anionic and cationic dyes
Abstract
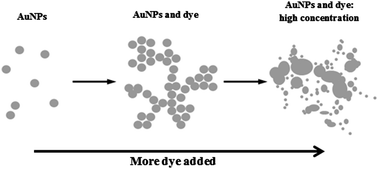
We describe the synthesis of charge-stabilised gold and silver nanoparticles by a modified Turkevich method and their interaction with a selection of cationic and anionic dyes. It was found that gold nanoparticles interact strongly with cationic dyes and in some cases enhanced absorption was observed by UV-visible spectroscopy. It is also shown that addition of cationic dyes to gold nanoparticles triggers aggregation of the nanoparticles into large, micrometre-scale clusters. Simultaneous fragmentation and agglomeration of the gold nanoparticles was observed at high concentrations of cationic dye in the solution. These effects were not observed when gold nanoparticles were mixed with anionic dyes, nor for silver nanoparticles with either cationic or anionic dyes.

 Please wait while we load your content...
Please wait while we load your content...