Kinetics study of solid ammonia borane hydrogen release – modeling and experimental validation for chemical hydrogen storage
Abstract
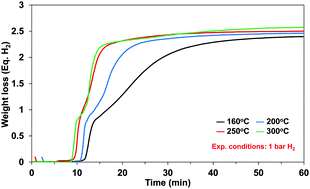
Ammonia borane (AB), NH3BH3, is a promising material for chemical hydrogen storage with 19.6 wt% gravimetric hydrogen capacity of which maximum 16.2 wt% hydrogen can be released via an exothermic thermal decomposition below 200 °C. We have investigated the kinetics of hydrogen release from AB and from an AB-methyl cellulose (AB/MC) composite at temperatures of 160–300 °C using both experiments and modeling. The hydrogen release rate at 300 °C is twice as fast as at 160 °C. The purpose of our study was to show safe hydrogen release without thermal runaway effects and to validate system model kinetics. AB/MC released hydrogen at ∼20 °C lower than neat AB and at a faster release rate in that temperature range. Based on the experimental results, the kinetics equations were revised to better represent the growth and nucleation process during decomposition of AB. We explored two different reactor concepts; auger and fixed bed. The current auger reactor concept turned out to not be appropriate, however, we demonstrated safe self-propagation of the hydrogen release reaction of solid AB/MC in a fixed bed reactor.

 Please wait while we load your content...
Please wait while we load your content...