Long-range proton-coupled electron transfer in phenol–Ru(2,2′-bipyrazine)32+ dyads†
Abstract
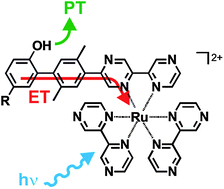
Two dyads in which either 4-cyanophenol or un-substituted phenol is connected via a p-xylene spacer to a Ru(bpz)32+ (bpz = 2,2′-bipyrazine) complex were synthesized and investigated. Selective photo-excitation of Ru(bpz)32+ at 532 nm in a CH3CN–H2O mixture leads to the formation of 4-cyanophenolate or phenolate along with Ru(bpz)32+ in its electronic ground state. This apparent photoacid behavior can be understood on the basis of a reaction sequence comprised of an initial photoinduced proton-coupled electron transfer (PCET) during which 4-cyanophenol or phenol is oxidized and deprotonated, followed by a thermal electron transfer event in the course of which 4-cyanophenoxyl or phenoxyl is reduced by Ru(bpz)3+ to 4-cyanophenolate or phenolate. Conceptually, this reaction sequence is identical to a sequence of photoinduced charge-separation and thermal charge-recombination events as observed previously for many electron transfer dyads, with the important difference that the initial photoinduced electron transfer process is proton-coupled. The dyad containing 4-cyanophenol reacts via concerted-proton electron transfer (CPET) whereas the dyad containing un-substituted phenol appears to react predominantly via a stepwise PCET mechanism. Long-range PCET is a key reaction in photosystem II. Understanding the factors that govern the kinetics of long-range PCET is desirable in the broader context of light-to-energy conversion by means of proton–electron separation across natural or artificial membranes.

 Please wait while we load your content...
Please wait while we load your content...