Reversible potentials for steps in methanol and formic acid oxidation to CO2; adsorption energies of intermediates on the ideal electrocatalyst for methanol oxidation and CO2 reduction
Abstract
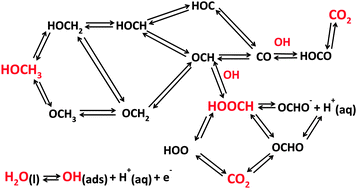
Quantum chemical theory is used to identify the reasons for platinum's limitations as an electrocatalyst for oxidizing methanol at fuel cell anodes. The linear Gibbs energy relation (LGER) method is employed to predict reversible potentials for reaction steps for intermediates on the electrode surface. In this procedure, standard reversible potentials are calculated for the reactions in bulk solution phase and then they are perturbed using calculated adsorption bond strengths to the electrode surface, yielding the equilibrium potentials for each electron transfer step for adsorbed intermediates. Adsorption properties of ideal electrocatalysts for the methanol oxidation are found by imposing the condition that the reversible potential of each electron transfer step equals that for the overall reaction. The adsorption bond strengths that provide the ideal properties also apply to formic acid oxidation and carbon dioxide reduction. It is instructive to think of the ideal electrocatalyst as a lens that focusses the reversible potentials for the n individual electron transfer steps to the reversible potential for the n-electron process. It is found that the ideal catalyst will adsorb many intermediates, including HOOC, CO, OCH, HOC, HOCH, HOCH2, and OCH3 more weakly than platinum, and OOCH and OH more strongly. For example, for one possible pathway it is necessary to weaken adsorption bond strengths for HOCH2, HOCH, OCH, HOOC by about 0.5 eV, weaken adsorption CO by about 1.1 eV and strengthen OH adsorption by about 0.6 eV. These results imply a need for developing new multi-component catalysts.

 Please wait while we load your content...
Please wait while we load your content...