Atomic electron affinities and the role of symmetry between electron addition and subtraction in a corrected Koopmans approach
Abstract
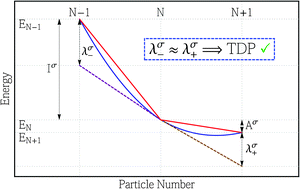
The essential aspects of zero-temperature grand-canonical ensemble density-functional theory are reviewed in the context of spin-density-functional theory and are used to highlight the assumption of symmetry between electron addition and subtraction that underlies the corrected Koopmans approach of Tozer and De Proft (TDP) for computing electron affinities. The issue of symmetry is then investigated in a systematic study of atomic electron affinities, comparing TDP affinities with those from a conventional Koopmans evaluation and electronic energy differences. Although it cannot compete with affinities determined from energy differences, the TDP expression yields results that are a significant improvement over those from the conventional Koopmans expression. Key insight into the results from both expressions is provided by an analysis of plots of the electronic energy as a function of the number of electrons, which highlight the extent of symmetry between addition and subtraction. The accuracy of the TDP affinities is closely related to the nature of the orbitals involved in the electron addition and subtraction, being particularly poor in cases where there is a change in principal quantum number, but relatively accurate within a single manifold of orbitals. The analysis is then extended to a consideration of the ground state Mulliken electronegativity and chemical hardness. The findings further emphasize the key role of symmetry in determining the quality of the results.
- This article is part of the themed collection: Density functional theory and its applications

 Please wait while we load your content...
Please wait while we load your content...