Crystallographic and computational study of 1-(arylamino)-1,2,3-triazole-4-carbohydrazides†
Abstract
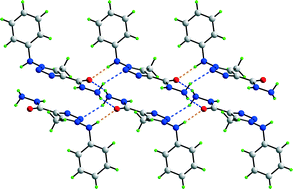
The crystallography of mono-p-substituted derivatives of 1-(arylamino)-1,2,3-triazole-4-carbohydrazides, 1 (X = H), 2 (F), 3 (Cl) and 4 (Br), and a 2,5-dichloro (5) analogue, shows the molecular structures to be similar. Distinct hydrogen bonding patterns based on N–H⋯N and N–H⋯O are observed in their crystal structures with 1, having two independent molecules comprising the asymmetric unit, displaying one pattern, 2 and 5 another, and 3 and 4 yet another. Geometry optimisation calculations indicate that any conformational differences in the solid state do not persist in the gas-phase and that no influence of the substituents is seen on the geometric parameters. A natural population analysis, for both experimental and optimised structures, shows that the charge on the triazole-N3 atom is at a maximum for 1, as opposed to 2–5, an observation correlated with its distinctive packing based around a supramolecular synthon not seen in the other structures. For the molecules having electronegative substituents, molecular electrostatic potentials show that the energies of the amine-H4n atoms are reduced for 2 and 5, compared to 3 and 4. A further distinction in 2–5 is indicated by the Hirshfeld surface analysis which highlights the importance of π⋯π interactions in 2 and 5, i.e. with the more electronegative substituents. Clearly, there is interplay between various factors but all correlated with the influence of the electronegativity of the substituent(s).

 Please wait while we load your content...
Please wait while we load your content...