Pharmaceutical cocrystals of the anti-tuberculosis drug pyrazinamide with dicarboxylic and tricarboxylic acids†
Abstract
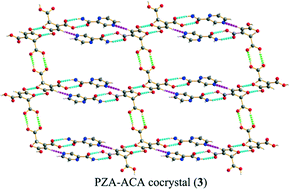
Four cocrystals of the anti-tuberculosis drug pyrazinamide (PZA) with adipic acid (1), sebacic acid (2), trans-aconitic acid (3), and citric acid (4) were successfully designed and synthesised. Their structures were determined by single-crystal X-ray diffraction, in which 1 and 2 displayed one-dimensional chain structures, while 3 and 4 formed two-dimensional hydrogen-bonded frameworks between PZA and the coformers. The equilibrium solubility and intrinsic dissolution rate (IDR) of the four cocrystals and PZA itself were then determined. The results demonstrate that 3 and 4 exhibit superior solubility and IDR relative to PZA.

 Please wait while we load your content...
Please wait while we load your content...