Relationships between the racemic structures of substituted mandelic acids containing 8- and 10-membered hydrogen bonded dimer rings†
Abstract
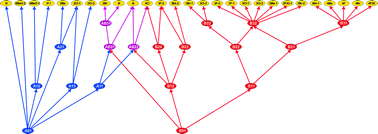
The structures of 27 monosubstituted mandelic acids, including several of their polymorphs, plus unsubstituted mandelic acid itself (two polymorphs) are investigated for structural similarity. The results, presented pictorially as a structural relationship plot, show that rather more structures are built up from the carboxyl-chain hydroxyl hydrogen bonded dimer than from the conventional carboxylic acid dimer. The results show how all the structures are related and, based on the two types of dimer, the degree of similarity that they possess. Some structures with Z′ > 1 contain both sorts of dimers and there are many examples of isostructural sets within the structures so far determined. We also present an example where analysing similarity in related families of structures highlights a structure that should be present and which has indeed then proceeded to be synthesised and determined.


 Please wait while we load your content...
Please wait while we load your content...