Selectivity assessment in host–guest complexes from single-crystal X-ray diffraction data: the cavitand–alcohol case†
Abstract
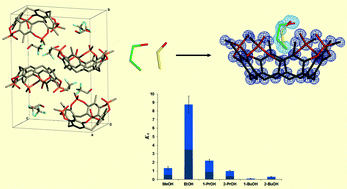
The determination of selectivity is central to the development of molecular receptors. Competition binding experiments based on the relative binding ratios are commonly used to evaluate relative binding constants. When more than one species binds to the same active site of a receptor, the crystallographic evaluation of the occupancy factors of each ligand could be very informative about their relative affinity. However, in the presence of overlapped electron densities, the statistical occupancy factors are hard to retrieve using conventional crystal structure refinement. Here, we present an original method to evaluate the relative binding constants based on the direct treatment of the diffraction intensities obtained from isomorphous single crystals grown in the presence of binary mixtures of competitive ligands. This method was developed and first applied to evaluate the affinity of a tetraphosphonate cavitand receptor towards short-chain alcohols. In the easier cases, wherein the electron densities of the two alcohols are less overlapped, the occupancy factors for guest molecules obtained in conventional structural refinement are in good agreement with the values calculated from the direct comparison of diffraction intensities. The affinity constants were estimated from the calculated occupancy factors, considering the molar ratios of alcohols used in the competition experiments. The congruence of the method has been tested on several binary mixtures using different concentration ratios of alcoholic guests. In general, for a given alcoholic pair, the different molar ratios of alcohols, used in the crystallization batch, produce a trend of the occupancy factors in agreement with the binding constant ratios of the two alcohols. From 32 data sets collected at the Elettra synchrotron for six short alkylic chain guest molecules, we evaluated the binding constant ratios with a good internal consistency. The relative binding constants for these six alcohols were evaluated from the entire statistical sample (EtOH, 8.8 > 1-PrOH, 2.2 > MeOH, 1.3 > 2-PrOH, 1.00 > 2-BuOH, 0.32 > 1-BuOH, 0.11, using 2-PrOH as reference with an arbitrary value of 1) and are in good agreement with the structural parameters of host–guest interactions observed in the corresponding crystal structures. In particular, the binding constant decreases with the increase in the host–guest H-bond distance, which follows the increase in the length of the alkyl chain of the alcoholic guest. Moreover, quartz crystal microbalance (QCM) measurements and fluorescence data have been compared and discussed with respect to the relative affinity scale obtained by crystallography.

 Please wait while we load your content...
Please wait while we load your content...