Biguanide and squaric acid as pH-dependent building blocks in crystal engineering†
Abstract
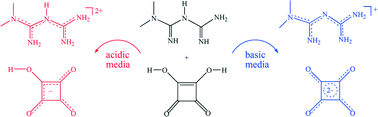
In solutions containing biguanides and squaric acid, the pH value allows to control the outcome of crystallization. From N,N-dimethylbiguanide, N-phenylbiguanide and N-o-tolylbiguanide 10 different salts have been obtained as a function of proton activity. The constituents in these ionic solids reflect the species present in solution: hydrogensquarate, still a strong acid, only occurs in crystals precipitated from acidic medium, and biguanidinium monocations prevail in precipitates from basic solutions. Within these limitations, polymorphs and hydrates of different stoichiometry have been encountered. The pH of the crystallization medium also affects the nature of close contacts. The shortest hydrogen bonds in the crystalline solids do not occur between ions of opposite charge; rather, pH matching leads to close O–H⋯O contacts between squarate and hydrogensquarate anions which can only coexist at pH < 4.

 Please wait while we load your content...
Please wait while we load your content...