Polymeric one- and two-dimensional copper(i) iodide complexes showing photoluminescence tunable by azaaromatic ligands†
Abstract
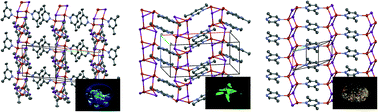
Four low-dimensional [(CuI)xL]n complexes [L = 4,6-dimethylpyrimidine (46dmpm; x = 2), 2,5-dimethylpyrazine (x = 2), 2,6-dimethylpyrazine (x = 1), 3,5-dimethylpyridine (x = 1)] were structurally characterized. All the compounds exhibited a (CuI)n polymeric motif and the azaaromatic ligands were coordinated to each copper(I) ion, thus giving infinite two-dimensional networks for the x = 2 compounds and one-dimensional chain structures for the x = 1 compounds. The photoluminescent properties were investigated in the solid state at ambient temperature, and blue, green, and orange emissions were observed for the pyridine, pyrimidine, and pyrazine compounds, respectively. The energy level of the π* orbital of the azaaromatic compounds regulates the emission color, in agreement with the halide-to-ligand charge-transfer mechanism. A photoluminescence quantum yield of 73% was recorded for [(CuI)2(46dmpm)]n.

 Please wait while we load your content...
Please wait while we load your content...