Mesoporous CeO2 nanoparticles assembled by hollow nanostructures: formation mechanism and enhanced catalytic properties†
Abstract
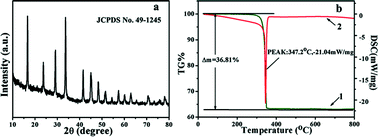
In this paper, novel hierarchically mesoporous CeO2 nanoparticles assembled by hollow nanocones were prepared through a facile solvothermal strategy using Ce(HCOO)3 as the precursor. X-Ray diffraction (XRD), transmission electron microscopy (TEM), high resolution transmission electron microscopy (HRTEM), field-emission scanning electron microscopy (FE-SEM) and thermal gravimetric analysis (TGA) were utilized to characterize the products and research the formation mechanism. The whole synthesis process involves two steps: formation of Ce(HCOO)3 nanoparticles constructed with nanocones at room temperature in an alkaline environment and oxidation induced phase transformation from Ce(HCOO)3 to CeO2 with formation of hollow nanocones assembled by nanocrystals in a solvothermal process at 150 °C. The as-prepared mesoporous CeO2 nanoparticles with an average diameter of 500 nm displayed a high surface area of 147.6 m2 g−1 using N2 adsorption and desorption measurement. The H2-TPR test showed its great reduction behavior in a low temperature zone. By comparing the T100 temperature of CO conversion with a commercial sample (above 350 °C) and other reported samples (above 300 °C) in the literature, the mesoporous CeO2 nanoparticles (270 °C) presented an excellent catalytic activity for CO oxidation.

 Please wait while we load your content...
Please wait while we load your content...