Isolation of a series of uranium organophosphinates†
Abstract
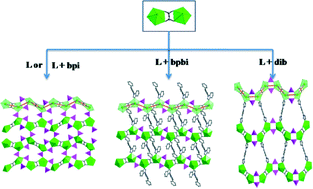
A new series of uranium organophosphinates has been hydrothermally synthesized by using hydroxymethyl phenylphosphinic acid (HMPPA, HL) in the presence of imidazole derivatives, namely, UO2(L)2 (HMPP-U1), (UO2)2(L)4(dib) (HMPP-U2), (UO2)2(L)2(ox)(dib) (HMPP-U3), (Hbpi)2[(UO2)4(L)6(ox)2] (HMPP-U4) and (UO2)2(L)2(ox)(bpbi)2 (HMPP-U5) (ox = oxalate, dib = 1,4-di(1H-imidazol-1-yl)benzene, bpi = 1-(biphenyl-4-yl)-1H-imidazole, bpbi = 1-([1,1′-biphenyl]-4-yl)-1H-benzo[d]imidazole). HMPP-U1 was directly synthesized through hydrothermal reaction between HMPPA and UO22+, featuring a single chain structure constructed from sole UO6 tetragonal bipyramids bridged by HMPP ligands. With further introduction of imidazole derivatives, a layered structure of HMPP-U2 is formed, in which dib molecules are coordinated to uranyl centers as bidentate coligands. By adding glycol into the synthesis, a common dimeric building unit is isolated, which comprises two U-centered pentagonal bipyramids linked by an in situ generated oxalate group. In HMPP-U3, a one-dimensional structure comprising such dimeric uranyl entities and HMPP ligands is pillared by dib, forming a layered assembly. HMPP-U4 incorporates the dimeric uranyl units connected by HMPP ligands, leading to a two-dimensional arrangement with bpi as the template. HMPP-U5 features a one-dimensional structure composed of the uranyl dimers and HMPP ligands. The bpbi molecules serving as coligands are coordinated to the chain. The syntheses and structures as well as the correlations and discrepancies of these uranyl organophosphinates are discussed in this paper.

 Please wait while we load your content...
Please wait while we load your content...