The effects of halogen bonding and molecular design on the self-assembly of coordination polymers of Mn(iii)-tetraarylporphyrin with axial bridging ligands†
Abstract
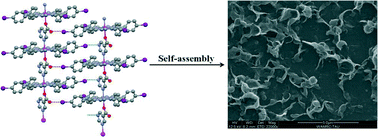
Two new Mn(III)-porphyrin 1D coordination polymers of the formula [MnIII(TIPP)(L)]n where TIPP = dianion of 5,10,15,20-meso-tetrakis(4-iodophenyl)porphyrin and L = isonicotinate (1) or pyrimidine-5-carboxylate (2) have been prepared through bridging heteroleptic coordination of the polydentate axial ligands. The two bridging ligands differ in the relative disposition and the number of molecular recognition sites. Single-crystal X-ray diffraction analysis reveals that directional O⋯I and N⋯I halogen-bonding-type interactions operate between neighboring polymeric chains in 2 but not in 1. Correspondingly, the SEM micrographs show completely different self-assembly patterns of the two complexes when deposited on a carbon fiber.

 Please wait while we load your content...
Please wait while we load your content...