Controlled synthesis of Co3O4 single-crystalline nanofilms enclosed by (111) facets and their exceptional activity for the catalytic decomposition of ammonium perchlorate†
Abstract
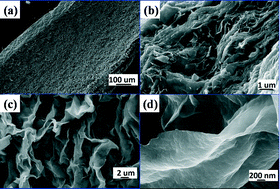
Single-crystalline, thermally stable Co3O4 (111) nanofilms have been successfully synthesized without any surfactant. The catalytic activity of Co3O4 nanofilms was investigated via the thermal decomposition of ammonium perchlorate (AP). AP exhibited unprecedentedly low decomposition temperatures and fast reaction rates in the catalyzed formations, making the Co3O4 (111) nanofilms an effective catalyst.

 Please wait while we load your content...
Please wait while we load your content...