Coordination polymers built with transition metal sulphates and angular 2,5-bis(imidazol-1-yl)thiophene (thim2): synthesis, structure and photoluminescent properties†
Abstract
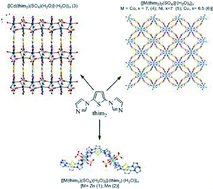
The reactions of angular tritopic ligand 2,5-bis(imidazol-1-yl)thiophene (thim2) with different metal sulphates afforded six new CPs {[M(thim2)(SO4)(H2O)2]2·(thim2)·(H2O)}n [M = Zn (1); Mn (2)], {[Cd(thim2)(SO4)(H2O)]·(H2O)}n (3) and {[M(thim2)2(SO4)]·(H2O)x}n [M = Co, x = 7, (4); Ni, x = 7 (5); Cu, x = 6.5 (6)]. CPs 1 and 2 are isostructural with a 1D double chain propagating in a wave like fashion. Whereas, CP 3 exhibits a layered 2D network and in the case of 4–6 an isostructural 3D framework containing a 1D rhombic channel was obtained. In all the CPs there is an interesting sulphate ion binding mode to the metal centers, such as four membered M2O4 rings (1 and 2), six membered (Cd2SO3) 1-D chain (3) and MSO4 (4–6) 1-D chain, which is further linked by thim2 to form inorganic organic hybrid type systems. The stability of CPs (1–6) was monitored by TGA and a solid state photoluminescence study was carried out for 1 and 3 at room temperature.

 Please wait while we load your content...
Please wait while we load your content...