Evolution of ZnO microstructures from hexagonal disk to prismoid, prism and pyramid and their crystal facet-dependent gas sensing properties†
Abstract
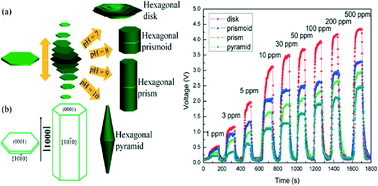
Herein, the evolution of ZnO structures from hexagonal disk to prismoid, prism and pyramid was found via a facile two-step low temperature hydrothermal reaction, and the evolution was achieved by only adjusting the pH value of the reactive solution without the assistance of a template or a surfactant. The characterization results showed that the precursor (hexagonal Zn5(OH)8Cl2·2H2O disk) played a key role in the morphology evolution of ZnO during the early stage of the growth process and that the disks tended to stack together layer by layer in both directions (up and down) to form prismoid, prism and pyramid structures with the increase in pH value from 7 to 10. After calcination, the corresponding hexagonal ZnO microstructures were obtained. This structure evolution resulted in the weakening dominance of the (0001) plane in the total exposed crystal facets. Furthermore, despite the similar specific surface areas of the four hexagonal ZnO microstructures, the gas sensing properties of the sensors based on these microstructures deteriorated sequentially. At a working temperature of 330 °C, the ZnO disk with the most exposed (0001) plane showed the highest gas response toward ethanol, which was nearly 2, 3, and 6 times higher than those of the prismoid, prism and pyramid structures, respectively. This superior gas sensing performance strongly depends on the predominantly exposed polar facets (0001), which can provide more active sites for oxygen adsorption and subsequent reaction with the detected gas than other apolar facets. It demonstrates that the (0001) crystal facet plays a significant role in the gas sensing behavior of ZnO. This research will bring some inspiration to researchers for the fabrication of a high performance ZnO gas sensor as well as other metal oxides.

 Please wait while we load your content...
Please wait while we load your content...