Solution-mediated phase transformation of uric acid dihydrate†
Abstract
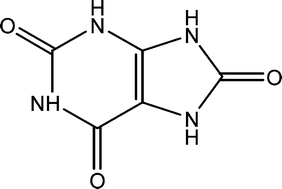
Various crystalline phases of uric acid are frequently identified components of human kidney stones, including anhydrous uric acid (UA) and uric acid dihydrate (UAD). Herein we report a quantitative study of the solution-mediated phase transformation of metastable UAD to UA as a function of pH as well as in model urine solution. Using a combination of X-ray diffraction, thermal analysis, and optical microscopy techniques, the UAD to UA transformation was found to go to completion within 48 hours at 37 °C in buffered solutions with pH between 4.0–6.5 with no evidence for intermediate crystalline phases. In solutions with pH > 6.8, UAD transformation to a different monosodium urate monohydrate phase becomes dominant. In artificial urine solution, the transformation occurs on a slightly faster timescale and results in smaller UA crystals. Seeding and saturation experiments indicate that the rate-limiting step in the overall transformation is the dissolution of UAD. The kinetics of these transformation processes suggest that interconversions between various solid state forms of uric acid are relevant under the physiologic conditions which lead to stone formation.
- This article is part of the themed collection: International Year of Crystallography Celebration: North America

 Please wait while we load your content...
Please wait while we load your content...