Hirshfeld surface analysis of crystal packing in aza-aromatic picrate salts†
Abstract
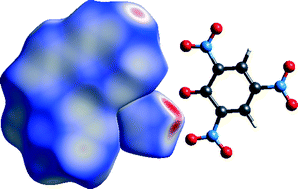
Single crystal X-ray structure determinations have previously been described for picrate salts of a variety of nitrogen bases. Herein, these have been extended to encompass monoprotonated mono- and oligo-dentate cyclic nitrogen-donor ligand systems derived from pyridine, some via saturation (piperidine and morpholine) and others via lateral extension of the aromatic system (2,2′-bipyridine, 1,10-phenanthroline, 2,9-dimethyl-1,10-phenanthroline, bis(2-pyridyl)amine, 2,2′:6′,2′′-terpyridine, and 8-hydroxyquinoline). Hydrogen-bonding interactions are dominant determinants of the structures, complemented by or in competition with parallel stacking of anion and (aromatic) base planes. Furthermore, nitro⋯nitro, nitro⋯π and phenoxy-O⋯π inter-species contacts play a significant role in the crystal packing. It also appears that cation⋯anion interactions arising from CH(adjacent to NH)⋯O(o-nitro) interactions are more important than the available secondary bifurcating component associated with any NH⋯O(o-nitro) approach, resulting, in many cases, in a bidentate NH⋯HC base approach to an (ON)O⋯O⋯O(NO) triadic array. The nature of the anion–cation interactions and their importance are explored using the Hirshfeld surface method. The precision of the structure determinations establishes the quinonoid form of the picrate to be a widespread contributor. Theoretical calculations on picric acid and the parent pyridinium–picrate ion-pair confirm the energetic favourability of the base-triad approach and the dominance of the quinonoid resonance form.

 Please wait while we load your content...
Please wait while we load your content...