Anatase TiO2 hollow nanosheets: dual roles of F−, formation mechanism, and thermal stability†
Abstract
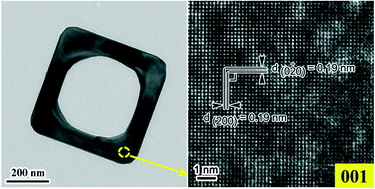
Anatase TiO2 hollow nanosheets with a width of 550 nm, a thickness of 100 nm, and a hole diameter of 350 nm were hydrothermally fabricated in an aqueous solution containing NH4VO3, HF, and HCl at an appropriate composition. Structural analyses on the products obtained at different intervals during the synthesis revealed that the formation of the anatase TiO2 hollow nanosheets consisted of three steps: oriented assembly of square-like NH4TiOF3 nanoparticles, topochemical conversion of NH4TiOF3 to anatase TiO2, and selective etching by F− on the flat nanosheets. The fluorine anion was involved in the formation of NH4TiOF3 as the key intermediate, it directed the construction of the nanosheet, and participated in the etching process to generate the hollow structure. The resultant anatase TiO2 hollow nanosheets exhibited a rather high thermal stability, maintaining the anatase crystallite structure and the hollow shape up to 1073 K.

 Please wait while we load your content...
Please wait while we load your content...