Vanadium-doped TiO2 anatase nanostructures: the role of V in solid solution formation and its effect on the optical properties†
Abstract
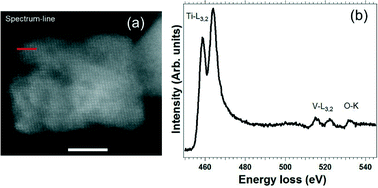
A facile and environmentally friendly synthesis approach for the production of vanadium doped titanium dioxide (VxTi1−xO2) nanostructures was demonstrated via hydrothermal decomposition of vanadium and titanium peroxo-complexes. The effect of vanadium addition on the structural and morphological properties of VxTi1−xO2 nanocrystals was investigated by X-ray diffraction (XRD) and electron microscopy techniques. XRD analysis showed that all VxTi1−xO2 samples presented only the TiO2 anatase crystalline phase and, despite the different amounts of vanadium ions, the single crystalline nature was preserved. Increasing V contents resulted in morphological evolution, from anisotropic to isotropic structures. X-ray absorption spectroscopy (XAS) and electron energy loss spectroscopy (EELS) have been employed for investigating the atomic composition and configuration of these nanostructures. XAS measurements at the K-edges (for V and Ti) revealed that V ions occupy the Ti4+-site, which confirms the doping effect. Furthermore, high-angle annular dark-field (HAADF) imaging, combined with EELS mapping, indicated that the vanadium ions were homogeneously distributed in the structure without any kind of segregation. These morphological and compositional modifications upon vanadium addition led to evolution of the TiO2 optical properties.

 Please wait while we load your content...
Please wait while we load your content...