Selective purification and chemical labeling of a target protein on ruthenium photocatalyst-functionalized affinity beads†
Abstract
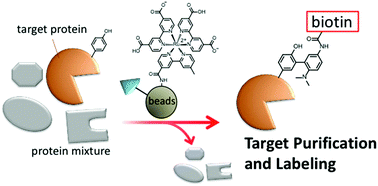
Selective purification and chemical labeling of a target protein in a protein mixture were simultaneously achieved on the surface of affinity beads functionalized with ligands, such as benzenesulfonamide and methotrexate (MTX), and a ruthenium complex containing 2,2′-bipyridine-4,4′-dicarboxylic acid (dcbpy). Chemical labeling of the target protein with a tyrosine radical trapper (TRT) proceeded on the surface of the beads when the target protein was in close proximity to the ruthenium photocatalyst. Both the protein purification and chemical labeling abilities of the affinity beads functionalized with ruthenium photocatalyst were not compromised after recycling several times. Dihydrofolate reductase (DHFR) endogenously expressed in HeLa cells was detected by chemical labeling with biotin-TRT on the affinity beads with high sensitivity compared to the conventional silver staining method.


 Please wait while we load your content...
Please wait while we load your content...