Chiral phosphoric acid catalyzed oxidative kinetic resolution of cyclic secondary amine derivatives including tetrahydroquinolines by hydrogen transfer to imines†
Abstract
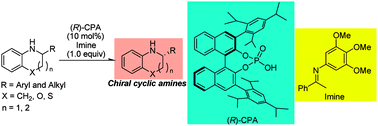
A chiral Brønsted acid catalyzed dehydrogenative kinetic resolution of tetrahydroquinoline derivatives, which are representative of cyclic secondary amines, based on their hydrogen transfer to aromatic imines was efficiently achieved with high enantioselectivities. This hydrogen transfer of tetrahydroquinolines to imines was not driven by their aromatization to quinolines. This dehydrogenative kinetic resolution could be also applied to the asymmetric synthesis of various benzofused heterocycles containing secondary amine cores.

 Please wait while we load your content...
Please wait while we load your content...