Identifying alkali metal inhibitors of crystal growth: a selection criterion based on ion pair hydration energy†
Abstract
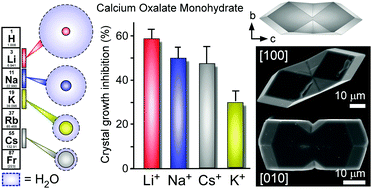
We show that alkali metals function as effective modifiers of calcium oxalate monohydrate (COM) crystallization wherein alkali-oxalate ion parings reduce the rate of crystal growth by as much as 60%. Our findings reveal a distinct trend in alkali metal efficacy that cannot be explained by colloidal theories or simple descriptors, such as ion size, but is consistent with a theoretical model that accounts for the ion pair's affinity for water.

 Please wait while we load your content...
Please wait while we load your content...