Thiophosphoramides as cooperative catalysts for copper-catalyzed arylation of carboxylates with diaryliodonium salts†
Abstract
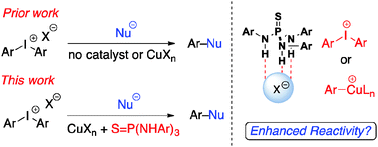
A thiophosphoramide-based co-catalyst was found to significantly accelerate copper(II) trifluoromethanesulfonate-catalyzed arylation of potassium carboxylates with diaryliodonium salts. This effect could be attributed to counterion activation of diaryliodonium salts or organocopper intermediates by thiophosphoramides. Inclusion of thiophosphoramides permits achieving significantly milder reaction conditions and expands the scope of solvents and diaryliodonium counterions that could be used for the arylation of carboxylate nucleophiles.
- This article is part of the themed collection: 2015 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...