Direct reductive coupling of secondary amides: chemoselective formation of vicinal diamines and vicinal amino alcohols†‡
Abstract
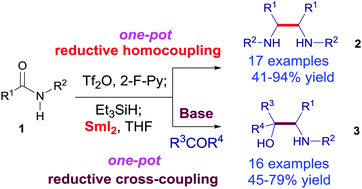
We report the first one-pot reductive homocoupling reaction of secondary amides and cross-coupling reaction of secondary amides with ketones to give secondary vicinal diamines and amino alcohols. This method relies on the direct generation of α-amino carbon radicals from secondary amides by activation with trifluoromethanesulfonic anhydride, partial reduction with triethylsilane and samarium diiodide-mediated single-electron transfer. The reactions were run under mild conditions and tolerated several functional groups.

 Please wait while we load your content...
Please wait while we load your content...