A temporary-bridge strategy for enantioselective organocatalyzed synthesis of aza-seven-membered rings†
Abstract
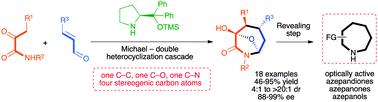
We report the first enantioselective organocatalyzed domino synthesis of azepane moieties. This temporary-bridge strategy is based on a conceptually original annulation of ambident electrophilic and 1,4-bis-nucleophilic α-ketoamides with 1,3-bis-electrophilic enals. The obtained oxygen-bridged azepanes can be selectively transformed into optically active azepanone, azepanol or azepanedione derivatives of high synthetic value.

 Please wait while we load your content...
Please wait while we load your content...