Introducing deep eutectic solvents as biorenewable media for Au(i)-catalysed cycloisomerisation of γ-alkynoic acids: an unprecedented catalytic system†
Abstract
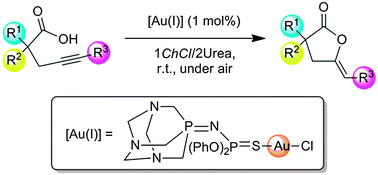
Cycloisomerisation of γ-alkynoic acids into cyclic enol-lactones was conveniently performed, for the first time, in the eutectic mixture 1ChCl/2Urea under standard bench experimental conditions (at room temperature, under air and in the absence of co-catalysts) by using a new iminophosphorane–Au(I) complex as the catalyst. Furthermore, the catalytic system could be recycled up to four runs.

 Please wait while we load your content...
Please wait while we load your content...