Chemoselective silyl transfer in the Mukaiyama aldol reaction promoted by super silyl Lewis acid†
Abstract
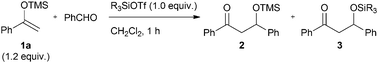
In the silyl Lewis acid-promoted Mukaiyama aldol reaction, the steric and electronic properties of the silyl group on the silyl Lewis acid influence the reaction mechanism and product distribution. When super silyl triflates such as (TMS)3SiOTf and (TES)3SiOTf are used as Lewis acids, the silyl group of the silyl enol ether chemoselectively transfers to the product. The mechanistic details have been investigated using density functional theory (DFT) calculations.

 Please wait while we load your content...
Please wait while we load your content...