An organocatalytic domino Michael-alkylation reaction: highly enantioselective construction of spiro-cyclopentanoneoxindoles and tetronic acid scaffolds†
Abstract
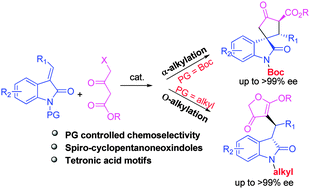
A new organocatalytic asymmetric domino Michael-alkylation reaction of methyleneindolinones and γ-halogenated-β-ketoesters is described. A variety of spiro-cyclopentanoneoxindoles were obtained in high yields (up to 96%), good diastereoselectivities (up to 12 : 1 dr) and excellent enantioselectivities (up to >99% ee) via α-alkylation. Interestingly, O-alkylated products with tetronic acid motifs could be obtained by tuning the N-protecting groups on methyleneindolinones with excellent enantioselectivities (up to >99% ee).

 Please wait while we load your content...
Please wait while we load your content...