A drastic substituent effect on the emission properties of quinone diimine models and valuable insight into the excited states of emeraldine†
Abstract
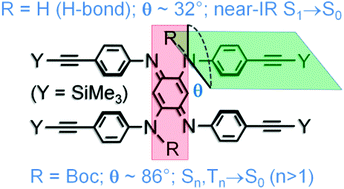
The (α-NR,α′-NR,N,N′-(C6H4C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3)4)[Q] models ([Q] = –N
CSiMe3)4)[Q] models ([Q] = –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6H4
C6H4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) exhibit upper excited state emissions Sn,Tn → S0 (n >1, R = Boc), similar to emeraldine, vs. a fluorescence S1 → S0 (R = H), driven by a large change in dihedral angles made by the NR–C6H4 and [Q] planes and intramolecular H-bonds.
N–) exhibit upper excited state emissions Sn,Tn → S0 (n >1, R = Boc), similar to emeraldine, vs. a fluorescence S1 → S0 (R = H), driven by a large change in dihedral angles made by the NR–C6H4 and [Q] planes and intramolecular H-bonds.

 Please wait while we load your content...
Please wait while we load your content...