A short synthetic route towards merosesquiterpenes with a benzoxanthene skeleton†
Abstract
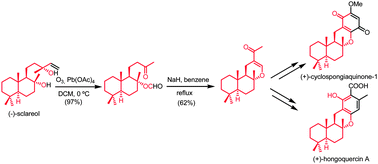
A short synthetic sequence for the preparation of merosesquiterpenes with a benzoxanthene skeleton starting from commercial (−)-sclareol is reported. The D ring of the target compound is obtained through a Diels–Alder cycloaddition, involving a dienoldiether derived from a tricyclic α,β-enone synthesized in two steps from the starting diterpene. Utilizing this procedure, the preparation of (+)-hongoquercin A and the first synthesis of (+)-cyclospongiaquinone-1 were achieved.

 Please wait while we load your content...
Please wait while we load your content...