Supramolecular chirality in peptide microcrystals†
Abstract
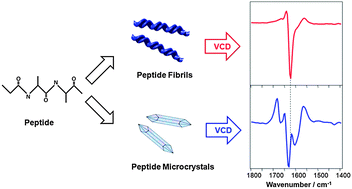
The vibrational circular dichroism (VCD) spectra of microcrystals of fibril-forming peptides have been measured for the first time. VCD spectra were measured and compared for microcrystals and fibrils formed from the same peptide, human islet amyloid polypeptide (IAPP, amylin). Structural information related to the supramolecular chirality of both the microcrystals and the fibrils, as well as the VCD enhancement mechanisms in fibrils and microcrystals, is obtained from these spectral comparisons. It is concluded that strongly enhanced VCD does not require braiding of two or more filaments that is permitted in fibrils but not microcrystals.

 Please wait while we load your content...
Please wait while we load your content...