A lithiomethyl trimethylammonium reagent as a methylene donor†
Abstract
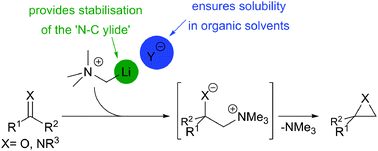
Straightforward deprotonation of soluble tetramethylammonium salts with alkyllithium reagents gives lithiomethyl trimethylammonium reagents. Coordination of the Li cation is crucial to the stability of these ‘N–C ylides’. These reagents were used to prepare epoxides, aziridines and allylic alcohols.

 Please wait while we load your content...
Please wait while we load your content...