Carbonylative enantioselective meso-desymmetrization of cis-epoxides to trans-β-lactones: effect of salen-ligand electronic variation on enantioselectivity†
Abstract
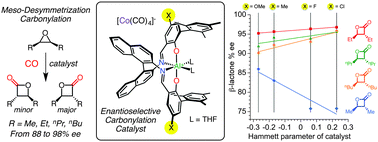
Carbonylation catalysts for the desymmetrization of meso-epoxides yielding enantioenriched trans-β-lactones are reported. Fine-tuning the electronic properties of the ligand further improved enantioselectivity. The sterics of the substrate dictated whether a given electronic variation decreased or increased enantioenrichment, revealing an unexpected relationship between the substrate's steric environment and the electronic nature of the optimal catalyst.

 Please wait while we load your content...
Please wait while we load your content...