A phosphine-mediated stereocontrolled synthesis of Z-enediynes by a vicinal dialkynylation of ethynylphosphonium salts†
Abstract
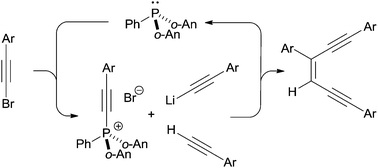
Z-Enediynes are prepared by a vicinal dialkynylation of triaryl(arylethynyl)phosphonium cations. The method, which proceeds under mild transition metal-free conditions, can be conducted on multigram scale as a one-pot, phosphine-mediated synthetic cycle giving enediyne products with excellent control of configuration.

 Please wait while we load your content...
Please wait while we load your content...