An electron transfer series of octahedral chromium complexes containing a redox non-innocent α-diimine ligand†
Abstract
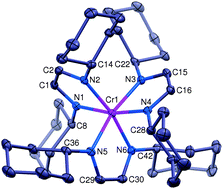
An electron-transfer series of octahedral α-diimine complexes [(HLCy)3Cr]n+(BARF)n (n = 2, 1, 0) has been synthesized and crystallographically characterized. Cyclic voltammetry indicated additional formation of [(HLCy)3Cr]3+. The molecular structures suggested that all redox processes were ligand-based. Magnetic moments were consistent with spin ground states of S = 0 for [HLCy3Cr]0, S = 1/2 for [HLCy3Cr]+1, and S = 1 for [HLCy3Cr]+2. The experimental data is consistent with chromium maintaining the +III oxidation state throughout, while being coordinated by varying numbers of neutral diimines (HLCy) and diimine radical anions (HLCy˙−).
- This article is part of the themed collection: Non-Innocent Ligands

 Please wait while we load your content...
Please wait while we load your content...