Highly effective copper-mediated gem-difluoromethylenation of arylboronic acids†
Abstract
A copper-mediated gem-difluoromethylenation of aryl, heteroaryl and vinyl boronic acids with bromodifluoromethylated oxazole or thiazole derivatives has been developed. This novel reaction showed an excellent functional group tolerance and wide substrate scope, providing facile access to practical application in drug discovery and development.
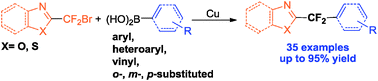

 Please wait while we load your content...
Please wait while we load your content...