Turning unreactive copper acetylides into remarkably powerful and mild alkyne transfer reagents by oxidative umpolung
Abstract
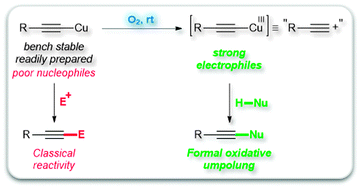
This is not breaking news: copper acetylides, readily available polymeric rock-stable solids, have been known for more than a century to be unreactive species and piteous nucleophiles. This lack of reactivity actually makes them ideal alkyne transfer reagents that can be easily activated under mild oxidizing conditions. When treated with molecular oxygen in the presence of simple chelating nitrogen ligands such as TMEDA, phenanthroline or imidazole derivatives, they are smoothly oxidized to highly electrophilic species that formally behave like acetylenic carbocations and can therefore be used for the mild and practical alkynylation of a wide range of nitrogen, phosphorus and carbon nucleophiles.

 Please wait while we load your content...
Please wait while we load your content...