Chemoselective arylation of phenols with bromo-nitroarenes: synthesis of nitro-biaryl-ols and their conversion into benzofurans and carbazoles†
Abstract
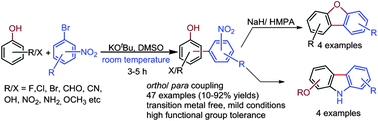
A series of electron withdrawing or donating group substituted phenols were chemoselectively arylated with various substituted bromo-nitroarenes using KOtBu at room temperature via an SNAr pathway. The synthesis of natural alkaloids (carbazoles), dibenzofurans, and a biaryl-indole was achieved from the synthesized nitro-biaryl-ols.

 Please wait while we load your content...
Please wait while we load your content...