Rhodium-catalyzed direct coupling of biaryl pyridine derivatives with internal alkynes†
Abstract
Axially chiral biaryls were synthesized by an isoquinoline or 2-pyridine-directed Rh(III)-catalyzed dual C–H cleavage and coupling with internal alkynes in good to excellent yields. Oxidation of isoquinoline derivatives with m-CPBA furnished their corresponding N-oxides, which could be utilized as Lewis base catalysts in asymmetric reactions.
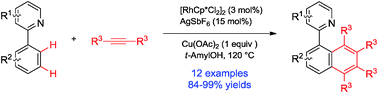

 Please wait while we load your content...
Please wait while we load your content...